Click here to start your patients
on the path to just 2 DOSES A YEAR
- For US Healthcare Professionals Only
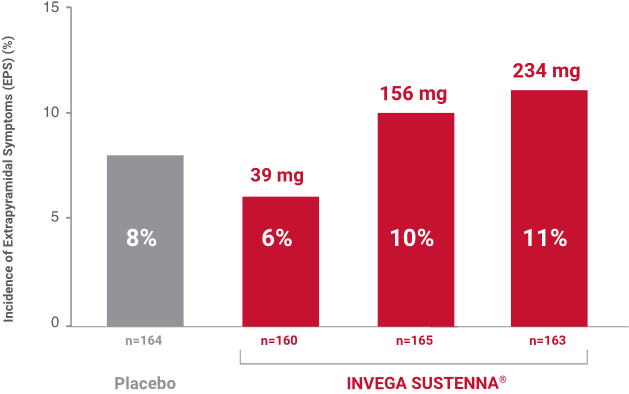
Results from a double-blind, randomized, placebo-controlled, fixed-dose, 13-week study of adult inpatients experiencing an acute exacerbation of schizophrenia. Patients were randomized to receive placebo or a 234-mg deltoid injection initiation dose on Day 1, followed by a 39-mg, 156-mg, or 234-mg dose in either the deltoid or gluteal muscle on Day 8, and once-monthly thereafter.1,2
Pooled data from two 13-week, double-blind studies showed that the overall percentages of EPS-related adverse events were 10% in the placebo group and 12%, 11%, and 11% in the INVEGA SUSTENNA® (paliperidone palmitate) 39-mg, 78 mg, and 156-mg groups, respectively.1,3-5
In a 9-week, double-blind study, the incidence of parkinsonism and akathisia was higher in the INVEGA SUSTENNA® 156-mg group (18% and 11%, respectively) than in the INVEGA SUSTENNA® 78-mg group (9% and 5%, respectively) and placebo group (7% and 4%, respectively).1,3,5
The results across all phases of the maintenance trial exhibited comparable findings.1
References: 1. INVEGA SUSTENNA® [Prescribing Information]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; July 2022. 2. Pandina GJ, Lindenmayer J-P, Lull J, et al. A randomized, placebo-controlled study to assess the efficacy and safety of 3 doses of paliperidone palmitate in adults with acutely exacerbated schizophrenia. J Clin Psychopharmacol. 2010;30(3):235-244. 3. Kramer M, Litman R, Hough D, et al. Paliperidone palmitate, a potential long-acting treatment for patients with schizophrenia. Results of a randomized, double-blind, placebo-controlled efficacy and safety study. Int J Neuropsychopharmacol. 2010;13(5):635-647. 4. Gopal S, Hough DW, Xu H, et al. Efficacy and safety of paliperidone palmitate in adult patients with acutely symptomatic schizophrenia: a randomized, double-blind, placebo-controlled, dose-response study. Int Clin Psychopharmacol. 2010;25(5):247-256. 5. Nasrallah HA, Gopal S, Gassmann-Mayer C, et al. A controlled, evidence-based trial of paliperidone palmitate, a long-acting injectable antipsychotic, in schizophrenia. Neuropsychopharmacol. 2010;35(10):2072-2082.